Archimedes' Principle
Background Reading
Science historians generally agree that the idea for his principle occurred to
Archimedes as he entered a bath pool. As he waded into deeper water, the
force on his feet became less.
Archimedes' Principle is that an object totally or partially immersed
in a fluid (liquid or gas) is buoyed (lifted) up by a force equal to the
weight of the fluid that is displaced.
It has numerous applications, one of which is the determination of density
and specific gravity. In the following discussion, the subscripts
S, W, and A represent the Substance, Water,
and Air, respectively.
Density is the mass per unit volume of a substance.
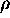 = M / V
= M / V
Specific Gravity is the ratio of the density of the substance to the
density of water.
SG = 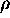 S /
S /
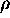 W.
W.
The metric system unit GRAM is defined to be the mass of one cubic
centimeter (one milliliter) of pure water at 3.98oC. Thus, for
water
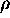 W
= 1 gram/cm3
and, if errors due to impurities and/or
temperatures are tolerable, this is a great convenience. The definitions
given above suggest various methods for determining specific gravity.
W
= 1 gram/cm3
and, if errors due to impurities and/or
temperatures are tolerable, this is a great convenience. The definitions
given above suggest various methods for determining specific gravity.
SG = 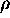 S /
S /
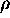 W
= (MS / VS) / (MW / VW)
= (MSg / VS) / (MWg / VW)
= (WS / VS) / (WW / VW)
W
= (MS / VS) / (MW / VW)
= (MSg / VS) / (MWg / VW)
= (WS / VS) / (WW / VW)
The definition of SG was applied for the first equality; the definition of
density was applied for the second equality. For the third equality, the
numerator and denominator were multiplied by "g". WS is the
weight of the substance measured in air; WW is the weight of the
water displaced by the substance when it is immersed.
In some circumstances, the volume of the substance is equal to the volume of
the water. In particular, when a solid object is completely immersed
in water, the volume of the water displaced must be equal to the volume of
the object. Furthermore, by Archimedes' Principle, upon immersion the
object would receive a buoyant force equal to the weight of the water
displaced. Thus, an object weighed in air and then weighed while immersed
in water would have an effective weight that was reduced by the weight
of the water displaced, if the buoyant force of the air is negligible.
When weighed in air, the object receives a buoyant force equal to the weight
of the air displaced by the object. However, the density of air is small
enough (compared to the density of most solids) to allow this buoyant force
to be neglected when weighing most solids in air.
(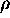 air
= 1.3 x 10-3 grams/cm3)
air
= 1.3 x 10-3 grams/cm3)
For an object more dense than water
SG = (WS / V) / (WW / V)
= WS / WW = WS / (buoyant force)
= WS / (loss of weight in water)
= WS / (WS - weight of substance in water)
This suggests a method for determining the SG of an object more dense than
water; namely, weight an object in air and weigh it while completely
immersed in water. The SG would then be the weight in air divided by the
apparent loss of weight when weighed in water.
A device called a Jolly Balance
is designed to measure the weight of
objects in air and in water by reading the elongation of a spring. It
utilizes a Vernier scale to read the elongation of the spring to the nearest
0.05 mm, and so is very precise. Hooke's law
F = - k x
should apply to the spring, where F is the force (weight) that stretches the
spring by an amount x, and k is the spring constant. Substituting into the
equation above, we find
SG = WS / (WS - weight of substance in water)
= (k xA) / (k xA - k xW)
When the unknown spring constant is canceled, the SG may be found using only
the two spring elongations
- xA: the object in air
- xW: the object completely submerged in water
SG = xA / ( xA - xW)
For an object less dense than water
The equation above is only true if the object is more dense than water. If
the object is less dense than water, a lead weight must be attached and three
spring elongations must be measured to determine the SG. The three
elongations are
- xA: the object alone, in air
- xB: the object in air with the lead sinker submerged
completed in water
- xC: both the object and the sinker completely submerged in water
The latter two elongations are used to determine the loss of weight in water,
that is, the denominator of the SG definition. You should derive this
formula in terms of xA, xB, and xC.
SG = WS / (loss of weight in water) = ?
Having determined the SG for sinking and floating objects using Archimedes'
Principle and the Jolly Balance, it is desirable to use an independent
method for comparison. Since
SG = 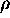 S /
S /
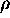 W,
and since
W,
and since 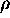 W
= 1 gram/cm3), one can determine the density of the object by
measuring its mass and volume directly.
W
= 1 gram/cm3), one can determine the density of the object by
measuring its mass and volume directly.
For a liquid
The Volumetric Flask (or Pycnometer) has a hollow stem stopper
that allows one to prepare equal volumes of fluids very reproducibly. If the
mass of the flask is measured (1) when empty, (2) when filled with the fluid,
and (3)when filled with water, the SG of the fluid can be determined. Since
the volume is the same, it will cancel out of the SG fraction.
Procedure
We will perform three separate experiments in which we will determine the
density and specific gravity of
- a solid more dense than water
- a solid less dense than water
- a liquid
Set up the Jolly Balance
- Level the Jolly balance using the screws on the legs so that the
scribed rod does not touch the clear plastic sleeve.
- Set the Vernier scale to zero (0.00 cm) using the knob near the base.
- Move the clear plastic sleeve until the scribe line on it is aligned
with the scribe line on the hanging metal rod.
- The Jolly balance is now calibrated to read zero when there is no load
on the spring, so the clear plastic sleeve MUST NOT BE MOVED
for the duration of the experiment.
Part 1- an object more dense than water
- Measure the mass of the dense (dark colored) ball on a
triple-beam balance.
- Measure the diameter of the ball using a
Digital caliper several times.
You decide how many. Choose many different orientations, not all in
the same plane. (Why?)
- Place the ball on the spring of the Jolly balance.
- Turn the knob at the base until the scribe line on the metal rod is
aligned with the scribe line on the plastic sleeve.
- Record the spring elongation xA.
- Fill the large metal beaker with water to about 3/4 capacity and place
it on the support table.
- Next, the ball should be completely submerged and the scribe line on the
metal rod should be aligned with the scribe line on the plastic sleeve.
To achieve this, adjust the spring elongation using the knob at the base
of the Jolly balance, and adjust the height of the support table, or
adjust the level of water in the large beaker by filling it from the
small beaker.
- Record the spring elongation xW.
Part 2 - an object less dense than water
- Measure the mass of the floating (light colored) ball on a
triple-beam balance.
- Measure the diameter of the ball using a
Digital caliper several times.
You decide how many. Choose many different orientations, not all in
the same plane. (Why?)
- Place the ball on the spring of the Jolly balance.
- Turn the knob at the base until the scribe line on the metal rod is
aligned with the scribe line on the plastic sleeve.
- Record the spring elongation xA.
- Attach the lead sinker to the bottom of the floating ball.
- Immerse all of the lead sinker, but none of the floating ball.
- Record the spring elongation xB.
- Immerse both the lead sinker and the floating ball.
- Record the spring elongation xC.
Part 3 - a liquid
- Measure the mass of the clean, dry pycnometer.
- Fill the pycnometer to the top with alcohol from the "clean" bottle
using a squeeze-bulb pipette.
Be certain to use the pipette marked "ALCOHOL" to avoid cross
contamination.
- Insert the hollow stem stopper. Notice that some fluid will be forced
out through the hollow stem. The volume of fluid in the pycnometer is
now very reproducible; the pycnometer can be filled to the same level
(namely the top of the hollow stem) with great precision.
- Dry any alcohol that may have spilled on the outside of the pycnometer.
- Measure the mass of the pycnometer filled with alcohol.
- Spill the used alcohol into the "waste" bottle.
- Rinse the pycnometer with water several times and fill it to the top
using the pipette marked "WATER".
- Insert the hollow stem stopper. Notice that some fluid will be forced
out through the hollow stem.
- Dry any water that may have spilled on the outside of the pycnometer.
- Measure the mass of the pycnometer filled with water.
- Spill the water into the sink and invert the pycnometer to dry.
- For pure isopropyl alcohol, SG = 0.810, but if this is cut with 30% water
then SG = 0.867. The latter should be used as the theoretical value for
comparison.
Error analysis
- Determine the SG of the dense ball using the Jolly balance measurements
with an error estimate.
- Determine the SG of the dense ball using the mass and volume measurements
with an error estimate.
- Compare the SG obtained by the two methods. Are they compatible?
- Determine the SG of the floating ball using the Jolly balance measurements
with an error estimate.
- Determine the SG of the floating ball using the mass and volume
measurements with an error estimate.
- Compare the SG obtained by the two methods. Are they compatible?
- Determine the SG of 70% isopropyl alcohol with an error estimate
and compare with the theoretical value. Is the theoretical value within
your error bars?
- Identify at least two sources of statistical error.
- Identify at least two sources of systematic error.
- The air is a fluid in which you and the experiment are immersed.
What is the fractional error incurred by neglecting the buoyant force
of the air on each of the wooden spheres? That is, what is the mass
of the displaced air divided by the mass you measured on the
triple-beam balance?
Justify the neglect of this buoyant force.
 Back to the Mechanics Manual
Back to the Mechanics Manual
= M / V
S /
W.
W
= 1 gram/cm3
and, if errors due to impurities and/or
temperatures are tolerable, this is a great convenience. The definitions
given above suggest various methods for determining specific gravity.
S /
W
= (MS / VS) / (MW / VW)
= (MSg / VS) / (MWg / VW)
= (WS / VS) / (WW / VW)
air
= 1.3 x 10-3 grams/cm3)
S /
W,
and since
W
= 1 gram/cm3), one can determine the density of the object by
measuring its mass and volume directly.
 Back to the Mechanics Manual
Back to the Mechanics Manual