The Bohr Model and the Hydrogen Spectrum
SPECIFIC OBJECTIVES
To understand the Bohr model of the hydrogen atom; to determine experimentally
the Rydberg constant.
EQUIPMENT
Spectrometer, diffraction grating, hydrogen light source,
high-voltage power supply.
BACKGROUND
Early spectroscopists discovered that every chemical element and compound
has a unique discrete bright-line spectrum which can be used to distinguish
it from all other elements and compounds. This is how we know, for example,
the composition of stars without ever having visited one for a sample of
material.
In 1885, through a feat of inductive reasoning, Balmer discovered, in a
totally empirical way, the formula for calculating the visible wavelengths
present in the hydrogen spectrum. That is, Balmer did not derive his formula
from a theory like Quantum Mechanics; he simply manipulated the numbers until
he found a pattern. A glance at the visible hydrogen wavelengths in the
table below will indicate the magnitude of his feat! Subsequently, after
much work attempting to explain why Balmer's formula gives the visible
hydrogen wavelengths so precisely, in 1913 Bohr provided a mathematical model
from which Balmer's formula could be derived. It is interesting to note that
28 years passed between Balmer's formula and the theoretical explanation for
it.
At first, Bohr's model was difficult to believe because one feature of the
model was so strange: the orbital electron in the hydrogen atom had certain
"permitted" orbits in which it would not emit light (even though electric
charges moving in circles must emit light according to the classical
electromagnetic theory). Bohr's electron would emit light, however, if it
"jumped" from one permitted orbit to another permitted orbit of lower energy.
(To jump from a low-energy orbit to a higher-energy orbit, the electron must
absorb light.)
Balmer's formula is
1 /  = R
(1 / n12 - 1 / n22 )
= R
(1 / n12 - 1 / n22 )
where R is called the Rydberg constant with value 109,677.58 cm-1,
n1=2 for the Balmer series of wavelengths, and n2 =
3,4,5, ... Each different value of n2 corresponds to a different
wavelength ( ) in the series.
) in the series.
Further Reading
Page 1,
Page 2,
Page 3,
Page 4.
PROCEDURE
Calibrating the Spectrometer
Check to ensure that the spectrometer is still calibrated by aligning the
hairline with the stationary side of the slit using the straight through
beam. The angle scale should read 0.0o.
Measuring
- CAUTION: The difraction grating is a photographic reproduction and
should NOT be touched. The deeper recess in the holder is intended
to protect it from damage. Therefore, the glass is on the shallow side of
the holder and the grating is on the deep side.
- Place the grating on the center of the table with its scratches running
vertically, and with the base material (glass) facing the light source.
In this way, one can study diffraction without the complication of
refraction (recall from the previous lab how light behaves when traveling
through glass at other than normal incidence). Fix the grating in place
using masking tape.
- Rotate the table to make the grating perpendicular to the incident beam
by eye. This is not critical since the average of
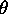 R and
R and 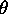 L accommodates a minor misalignment.
L accommodates a minor misalignment.
- Affirm maximum brightness for the straight through beam by adjusting the
source-slit alignment. At this step, the slit should be narrow, perhaps a
few times wider than the hairline. Search for the spectrum by moving the
telescope to one side or the other.
- For each of the four colors in the hydrogen spectrum, measure the
angles
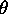 R and
R and
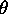 L to the
nearest tenth of a degree by placing the hairline on the stationary
side of the slit.
L to the
nearest tenth of a degree by placing the hairline on the stationary
side of the slit.
| Wavelength (angstroms) | Color | Intensity |
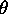 R R |
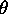 L L |
 |
| 6562.8 | Red | Strong |
| | |
| 4861.3 | Cyan | Strong |
| | |
| 4340.5 | Indigo | Strong |
| | |
| 4101.7 | Violet | Weak |
| | |

Analysis
- Average the right and left angles for each color.
- Use the grating equation with d = 1 / N to find the wavelength
 for each color, where
"N" is the grating constant determined experimentally in the previous
laboratory exercise.
for each color, where
"N" is the grating constant determined experimentally in the previous
laboratory exercise.
- Calculate the percent deviation for each wavelength using
% deviation = (data-theory)/theory x 100%
where "theory" is the tabulated wavelength. Do not ignore the sign;
it contains information. A positive % deviation means that the value
is above the theory; a negative % deviation means that the value is
below the theory.
- Use your experimental values of
 and the theoretical
value of the Rydberg constant (109,677.58 cm-1) in the Balmer
formula to determine n2 for each color. Remember that n2
must be an integer greater than 2. Round your experimental n2
to the nearest integer.
and the theoretical
value of the Rydberg constant (109,677.58 cm-1) in the Balmer
formula to determine n2 for each color. Remember that n2
must be an integer greater than 2. Round your experimental n2
to the nearest integer.
If all four of your n2 values are very nearly 2, you have performed
the unit conversion from angstroms to centimeters incorrectly.
Remember that 108 angstrom = 1 cm.
- Use your experimental values of
 and the integer
n2 values just found above in the Balmer formula to determine
an experimental value of the Rydberg constant for each of the four colors.
and the integer
n2 values just found above in the Balmer formula to determine
an experimental value of the Rydberg constant for each of the four colors.
- Report the best value of the Rydberg as the average of the four values
found above.
- Report the error on the best value of the Rydberg as the
standard deviation of the mean (SDOM). See Taylor section 4.4
if you are confused.
- Does your best experimental value of the Rydberg agree or disagree with the
theoretical value? See Taylor page 5 if you are confused.
Don't forget your two random and two systematic error sources.
 Back to the Electricity and Magnetism Manual
Back to the Electricity and Magnetism Manual

 Back to the Electricity and Magnetism Manual
Back to the Electricity and Magnetism Manual